前言 Introduction
噬菌体是地球上最丰富的生物实体,在其细菌宿主的生态和进化中起着关键作用。裂解(lytic)和溶原(lysogenic)途径是噬菌体生命周期的最佳描述。裂解途径是当噬菌体侵入细菌后,利用宿主菌的核糖体合成子代噬菌体,引起宿主细菌的裂解死亡,释放出子代噬菌体;溶原途径是溶原性噬菌体(温和性噬菌体)将DNA整合到宿主基因组,随细菌基因组复制而复制,并不裂解宿主菌。然而,发现越来越多的噬菌体可通过其他途径完成生命周期,使噬菌体经典的裂解-溶原途径受到了挑战。其中,假溶原(Pseudolysogeny)状态和携带者状态(carrier state)可能广泛的存在,并具有重要的生态作用,但当前对两者的研究不足和认识不够深入。因此,本文将围绕假溶原/携带者状态展开,从它们的定义、研究进展和研究挑战等几个方面进行简述,让大家认识到噬菌体-宿主菌相互作用的复杂性,远超经典的裂解-溶原途径,并引起足够的重视和促进相关的机制研究。
Phages are the most abundant biological entities on Earth and play a key role in the ecology and evolution of their bacterial hosts. The lytic and lysogenic pathways are the best descriptions of the phage life cycle. The lytic pathway occurs when a phage invades a bacterium and synthesizes a zygotic phage using the ribosome of the host bacterium, causing lysis and death of the host bacterium and releasing the zygotic phage; the lysogenic pathway occurs when a lysogenic phage (a mild phage) integrates its DNA into the host genome, replicates with the replication of the bacterial genome, and does not lyse the host bacterium. However, the discovery that an increasing number of phages can complete their life cycle through other pathways has challenged the classical lysis-lysis pathway of phages. Among them, the Pseudolysogeny (Pseudolysogeny) state and the carrier state may widely exist and have important ecological roles, but both are currently understudied and poorly understood. Therefore, this paper will focus on the pseudolysogeny/carrier state, and briefly describe their definitions, research progress and challenges, so as to recognize the complexity of phage-host bacterial interactions, which is much more than the classical lysis-lysis pathway, and to draw sufficient attention and promote the related mechanism research.
1 假溶原(Psedolysogeny)和携带者状态(carrier state)定义
1 Definition of Psedolysogeny and carrier state
1.1 回顾混乱的定义历程 1.1 Reviewing the Definitional History of Confusion
1963年,Stent[1]将假溶原状态的细菌描述为能吸附噬菌体颗粒,但对感染有抵抗力的细菌。Stent[1]进一步将假溶原状态的细菌描述为:细菌的细胞表面吸附有噬菌体颗粒,其中存在偶然的机会让噬菌体进入部分细菌细胞进行繁殖。几年后,Baess[2]给出了更详细的描述,他认为假溶原状态的细菌,噬菌体不整合到宿主菌的基因组上,而是以独立状态存在于细胞中,并且使用诱导溶原噬菌体的方法并不能将这些噬菌体诱导出来。而且,Baess[2]认为假溶原状态的细菌可以通过抗噬菌体血清培养或单菌落分离导致假溶原状态的消失。Barksdale&Arden[3]认为假溶原或者携带者状态都是噬菌体在部分细菌群体中繁殖的状态;而且,两者是由于敏感细菌细胞表面的受体数量减少或温和噬菌体自发突变为裂解状态的过程中导致了假溶原或者携带者状态的产生。同样,Ackermann&DuBow[4]将假溶原状态定义为培养中的细菌只有一部分被噬菌体感染,而且这些噬菌体不整合到宿主菌基因组中,而且可通过抗血清处理或亚克隆分离使假溶原状态消失。研究者认为这种相互作用在敏感细胞和抗性细胞的混合物或携带原噬菌体的细菌和烈性噬菌体的混合物中产生。Ackermann&DuBow[4]认为携带者状态是类似质粒状态的前噬菌体导致的溶原。Ripp&Miller[5,6]将假溶原状态描述为一种噬菌体-宿主菌相互作用的类型,其中噬菌体既没有整合到宿主染色体中也没有引起裂解,而是以非活动状态存在于细胞中。与溶原状态相反,假溶原状态的噬菌体基因组可以不与宿主染色体同步复制,导致噬菌体基因组以不对称方式遗传到子代细胞。而且,有趣的是,Ripp&Miller[5,6]认为假溶原状态需要特定的环境条件,比如在极度饥饿的情况下,由于没有足够的能量让噬菌体启动裂解或溶原周期;而且,随着环境条件的改善(获得更多可用的能量),假溶原状态将转换为裂解或者溶原状态。Ripp&Miller[5,6]认为假溶原状态让噬菌体在不利的环境条件下得以生存。Abedon[7]认为假溶原状态是生长不良的细菌携带了暂时不复制的噬菌体基因组,而携带者状态则指的是仅通过裂解部分细菌来维持噬菌体繁殖的状态。因此,噬菌体抗性和噬菌体敏感性细菌的基因可能不同,也可能相同,其取决于噬菌体不依赖或依赖机制产生的表型差异。
In 1963, Stent [1] described bacteria in the pseudolysogenic state as bacteria that adsorb phage particles but are resistant to infection.Stent [1] further described bacteria in the pseudolysogenic state as bacteria with phage particles adsorbed to their cell surfaces, where there exists serendipity for phages to enter some of the bacterial cells to multiply. A more detailed description was given a few years later by Baess [2], who argued that bacteria in the pseudolysogenic state have phages that are not integrated into the genome of the host bacterium but exist in the cell in an independent state, and that these phages are not induced using the method of inducing lysogenic phages. Moreover, Baess [2] suggested that bacteria in the pseudolysogenic state can be cultured in phage-resistant sera or single colony isolation leading to the disappearance of the pseudolysogenic state.Barksdale & Arden [3] suggested that either the pseudolysogenic or the carrier state is a state in which phages colonize a portion of the bacterial population; moreover, both are due to either a decrease in the number of receptors on the surface of the cell of the sensitive bacterium or to a mild phage spontaneous mutation to the lysogenic state results in the creation of the pseudolysogen or carrier state. Similarly, Ackermann & DuBow [4] defined the pseudolysogenic state as one in which only a portion of the bacteria in culture are infected by phages that do not integrate into the host bacterial genome, and where the pseudolysogenic state can be made to disappear by antiserum treatment or subclonal isolation. Researchers have suggested that this interaction arises in mixtures of sensitive and resistant cells or in mixtures of prophage-carrying bacteria and virulent phages.Ackermann&DuBow [4] suggested that the carrier state is a plasmid-like state of prophage leading to lysis.Ripp&Miller [5,6] described the pseudolysogenic state as a phage-host bacterial interaction type in which the phage neither integrates into the host chromosome nor causes lysis, but exists in the cell in an inactive state. In contrast to the lysogenic state, the phage genome in the pseudolysogenic state can replicate without synchronization with the host chromosome, resulting in the phage genome being inherited in an asymmetric manner into the daughter cells. Moreover, interestingly, Ripp & Miller [5,6] suggested that the pseudolysogenic state requires specific environmental conditions, such as under extreme starvation, due to the lack of sufficient energy for the phage to initiate a lytic or lysogenic cycle; and that, as the environmental conditions improve (with the acquisition of more available energy), the pseudolysogenic state will be converted to either a lytic or lysogenic state.Ripp & Miller [ 5,6] suggest that the pseudolysogenic state allows phages to survive under unfavorable environmental conditions.Abedon [7] suggests that the pseudolysogenic state is the result of poorly growing bacteria carrying a temporarily non-replicating phage genome, while the carrier state refers to a state in which phage reproduction is only sustained by lysing a portion of the bacteria. Thus, the genes of phage-resistant and phage-sensitive bacteria may be different or the same depending on phage-independent or mechanism-dependent phenotypic differences.
综上所述,研究人员早已认识到噬菌体-宿主相互作用的复杂性,超出了经典的裂解-溶原途径。然而,长期以来关于噬菌体假溶原/携带者状态的概念和术语上的混淆,犹如盲人摸象(图1),阻碍了这类超越经典的裂解-溶原途径的相关研究的进展。
In summary, researchers have long recognized the complexity of phage-host interactions beyond the classical lysis-lysogen pathway. However, the long-standing conceptual and terminological confusion regarding the phage pseudolysogen/carrier state, like a blind man feeling an elephant (Fig. 1), has impeded the progress of such studies related to the lysis-lysis pathway beyond the classical lysis-lysogen pathway.

图1 盲人摸象(图片来源于网络) Figure 1 Blind man feeling the elephant (image from the internet)
1.2 单细胞水平或群体水平进行重新定义 1.2 Redefinition at the single-cell level or at the population level
2021年,Mantynen[8]从单细胞水平定义了假溶原状态,认为其是一种常发生在细菌能量缺乏条件下的发育停滞状态,此时的噬菌体基因组不复制,并不对称地传递给子代细胞(表1;图2A)。同时,从群体水平定义了携带者状态,认为其是指噬菌体和细菌在群体水平上的稳定平衡,并且敏感细菌和抗性细菌的共存(表1;图2B)。假溶原/携带者状态被认为是在不利条件下维持噬菌体和细菌之间的平衡,能促进基因的水平转移。
In 2021, Mantynen [8] defined the pseudolysogenic state from the single-cell level as a state of developmental arrest that often occurs under conditions of bacterial energy deficiency, when the phage genome does not replicate and is asymmetrically passed on to progeny cells (Table 1; Figure 2A). Meanwhile, the carrier state was defined at the population level and considered to refer to a stable equilibrium between phage and bacteria at the population level and the coexistence of sensitive and resistant bacteria (Table 1; Fig. 2B). The pseudolysogen/carrier state is thought to maintain an equilibrium between phages and bacteria under unfavorable conditions that can facilitate horizontal gene transfer.
表1 假溶原/携带者状态的特性比较[8] Table 1 Comparison of characteristics of pseudolysogen/carrier states [8]
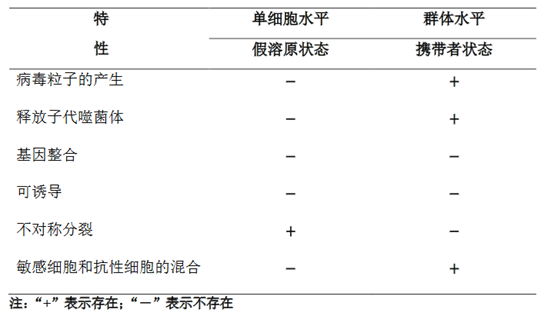
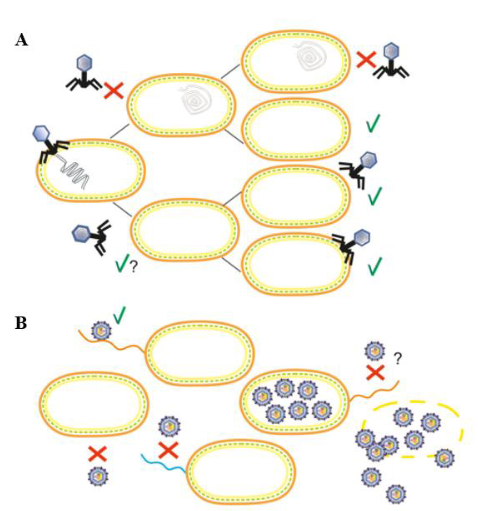
图2 假溶原/携带者状态的感染策略模式图[8]。(A)假溶原状态显示一个停滞的噬菌体发育阶段,其中未整合的噬菌体基因组不对称地传递给子代细胞。子代细胞可能通过噬菌体基因组的遗传或免疫因子(如噬菌体P22)对重复感染产生抗性(用红色“×”表示)。随后的细胞分裂稀释免疫因子,细菌亚群最终对噬菌体感染变得敏感(用绿色“√”表示)。(B)携带者状态描述噬菌体和细菌的混合物处于群体水平上的动态平衡,这是由于抗性细菌中存在敏感变异(易受噬菌体感染,因此容易发生噬菌体诱导裂解)。抗噬菌体的亚群可能是由宿主细胞的遗传和生理变化引起的。如图,噬菌体受体(野生型菌毛,橙色)的缺乏(无菌毛)或表型变化(菌毛突变体,蓝色)或细胞内噬菌体颗粒的存在诱导了噬菌体抗性的产生
Fig. 2 Diagram of infection strategy patterns for the pseudolysogen/carrier state [8]. (A) The pseudolysogen state shows a stagnant phage developmental stage in which the unintegrated phage genome is asymmetrically passed on to daughter cells. Daughter cells may become resistant to repeated infections (indicated by a red “×”) through inheritance of the phage genome or immune factors (e.g., phage P22). Subsequent cell divisions dilute the immune factors and the bacterial subpopulation eventually becomes susceptible to phage infection (indicated by a green “√”). (B) The carrier state describes a mixture of phages and bacteria in dynamic equilibrium at the population level due to the presence of sensitive variants (susceptible to phage infection and therefore prone to phage-induced lysis) in resistant bacteria. The phage-resistant subpopulation may be caused by genetic and physiological changes in the host cell. As shown, phage resistance is induced by the lack of phage receptors (wild-type hyphae, orange) (no hyphae) or phenotypic changes (hyphae mutants, blue) or the presence of intracellular phage particles
2 噬菌体假溶原/携带者状态的研究进展 2 Advances in the study of phage pseudolysogen/carrier status
近年来,在不同的细菌群体中发现了噬菌体假溶原/携带者状态的存在。很多文献仅描述了噬菌体载体状态的发现,并未对其进行机制研究,鲜有的几个例子进行了较深入的研究,具体详情如下:Connerton IF团队[9]发现,空肠弯曲杆菌(Campylobacter jejuni)中的一些噬菌体存在较稳定的携带者状态,烈性噬菌体可以持续与空肠弯曲杆菌结合,但并未发生基因组结合到宿主菌的基因组上,如图3所示。而且,研究发现处于携带者状态的空肠弯曲杆菌运动性减弱,可能是鞭毛基因的突变,损害了鞭毛运动和噬菌体吸附[10]。
In recent years, the presence of phage pseudolysogen/carrier states has been found in different bacterial populations. Much of the literature only describes the discovery of phage carrier states and does not investigate the mechanism, and few examples have been studied in more depth, as detailed below:The Connerton IF team [9] found that some phages in Campylobacter jejuni (C. jejuni) have a more stable carrier state, and potent phages can persistently bind to the Campylobacter jejuni but did not undergo genome binding to the genome of the host bacterium, as shown in Figure 3. Moreover, it was found that Campylobacter jejuni in the carrier state had diminished motility, possibly due to mutations in the flagellar genes that impair flagellar motility and phage adsorption [10].
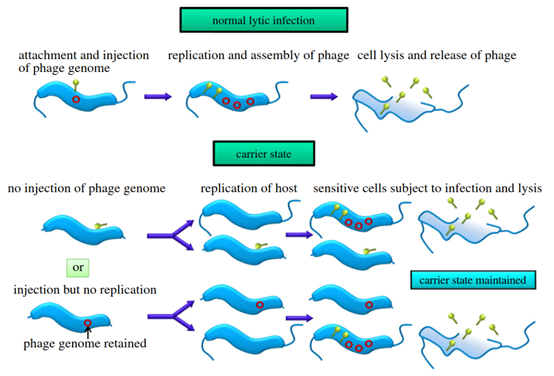 图3 空肠弯曲杆菌的裂解状态-携带者状态的感染过程示意图[8]
图3 空肠弯曲杆菌的裂解状态-携带者状态的感染过程示意图[8]
Fig. 3 Schematic diagram of the infection process of Campylobacter jejuni in the lysed state-carrier state [8]
Cenens W等人[11]在观察到鼠伤寒沙门菌(Salmonella typhimurium)的温和噬菌体P22进入溶原途径之前,存在短暂的携带者状态。研究发现,温和噬菌体P22通过这种策略,可以在感染群体中维持垂直-水平传播途径,而不会损害与宿主的稳定共存。同时,虽然高浓度的温和噬菌体P22通常被认为会推动其向宿主细胞的溶原转换,但Staes等人[12]发现温和噬菌体P22可以利用携带者状态的动力学和其重复感染排斥因子的表达,来提高宿主细胞亚群发生裂解(图4)。
Cenens W et al [11] observed a transient carrier state of the mild phage P22 of Salmonella typhimurium (S. typhimurium) before entering the lysogenic pathway. It was found that by this strategy, mild phage P22 could maintain the vertical-horizontal transmission pathway in the infected population without compromising stable coexistence with the host. Meanwhile, while high concentrations of mild phage P22 are usually thought to drive its lysogenic switch to host cells, Staes et al [12] found that mild phage P22 can utilize the kinetics of the carrier state and the expression of its repeat-infection rejection factor to enhance host cell subpopulations to undergo lysis (Figure 4).
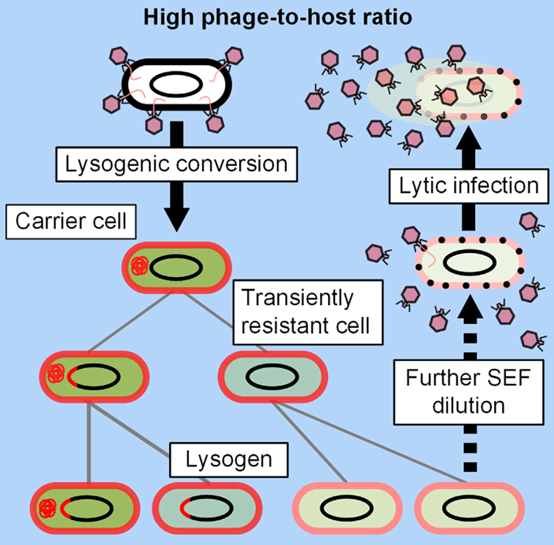
图4 重复感染排斥因子驱动温和噬菌体P22的垂直-水平传播转换示意图[11]
Fig. 4 Schematic diagram of vertical-horizontal propagation transition of mild phage P22 driven by repetitive infection rejection factors [11]
近期,王鹏/乐率团队[13]发现鼠疫耶尔森氏菌(Yersinia pestis)噬菌体HQ103存在携带者状态。而且,研究发现鼠疫菌编码的组蛋白样核结构蛋白H-NS在21℃高表达,以沉默该噬菌体的Cox启动子Pe,从而抑制其进入裂解周期;而在37℃时,由于鼠疫菌H-NS的减少,且该噬菌体抑制蛋白CI的截短以及启动子Pc的突变,从而使噬菌体HQ103从携带者状态转换成裂解状态,裂解鼠疫菌(图5)。同时,经土壤中细菌-噬菌体协同进化实验证实,在环境温度(21℃)下,噬菌体HQ103的携带者状态可以促进噬菌体-宿主菌共存(图5)。
Recently, Peng Wang/Le Lv team [13] found the existence of a carrier state for the phage HQ103 of Yersinia pestis (Yersinia pestis). Moreover, it was found that the histone-like nuclear structural protein H-NS encoded by Yersinia pestis was highly expressed at 21°C to silence the Cox promoter Pe of this phage, thus inhibiting its entry into the lysis cycle; whereas, at 37°C, due to the reduction of H-NS of Yersinia pestis and the truncation of the inhibitory protein CI of this phage as well as the mutation of the promoter Pc, which led to the conversion of phage HQ103 from the carrier state to the lysogenic state, lysing B. typhimurium (Figure 5). Meanwhile, the carrier state of phage HQ103 could promote phage-host bacterial coexistence at ambient temperature (21℃), as confirmed by the bacterial-phage coevolution experiment in soil (Figure 5).
图5 鼠疫菌编码的温度依赖性的组蛋白样核结构蛋白H-NS调控噬菌体HQ103裂解-携带者状态转换的机制示意图[13]
Fig. 5 Schematic representation of the mechanism by which the temperature-dependent histone-like nuclear structural protein H-NS encoded by B. plutonii regulates the phage HQ103 lysate-carrier state transition [13]
3 噬菌体假溶原/携带者状态的研究挑战 3 Research Challenges in Phage Pseudolysogen/Carrier Status
整体上来说,当前对噬菌体的假溶原/携带者状态的了解和研究十分有限,可归因于以下几个主要原因。首先,由于各种技术困难和缺乏明确定义的特征,识别它们具有挑战性。其次,当前认为噬菌体的假溶原/携带者状态往往相对不稳定,甚至认为假溶原/携带者状态的产生需要使用特殊的设备,如恒化器或发酵罐,与标准实验室条件有很大差异,从而增大了发现的难度,也使它们的研究变得复杂。最后,细菌群体中可能同时存在不止一种噬菌体感染模式,目前常用的噬菌体分离检测方法,更容易检测到噬菌体经典的溶原/裂解状态,而掩盖了噬菌体的假溶原/携带者状态的发现。
Overall, the current understanding and study of the pseudolysogen/carrier status of phages is limited and can be attributed to several main reasons. First, recognizing them is challenging due to various technical difficulties and lack of clearly defined characteristics. Second, the current belief that pseudolysogen/carrier states of phages tend to be relatively unstable, or even that the production of pseudolysogen/carrier states requires the use of special equipment, such as constantisers or fermentation tanks, that differ significantly from standard laboratory conditions, thus making their discovery more difficult and complicating their study. Finally, more than one phage infection mode may coexist in a bacterial population, and currently commonly used phage isolation assays are more likely to detect the classical lysogenic/lytic state of the phage, masking the discovery of the pseudolysogenic/carrier state of the phage.
结语 Epilogue
近些年,越来越多的噬菌体呈现假溶原/携带者状态,体现了噬菌体假溶原/携带者状态的普遍性,其具有重要的生态学意义,挑战了传统的裂解-溶原途径。但是,基于它们可能是条件依赖的,短暂的或仅发生在受感染宿主细菌群体的一部分,处于假溶原/携带者状态的噬菌体经常被忽视,局限了学者对它们的认识和重视,希望本文的简述能引起学者的关注和促进相关研究。
In recent years, an increasing number of phages have assumed the pseudolysogen/carrier state, reflecting the ubiquity of phage pseudolysogen/carrier states, which are ecologically important and challenge the traditional lysis-lysis pathway. However, based on the fact that they may be condition-dependent, transient or occur only in a part of the infected host bacterial population, phages in the pseudolysogen/carrier state are often overlooked, limiting scholars’ knowledge and attention to them, and it is hoped that the brief description in this paper will draw scholars’ attention and promote related research.
- 参考文献 bibliography
[1] Stent GS. Bacterial Viruses. (Book Reviews: Molecular Biology of Bacterial Viruses). Science, 1964. 143.
[2] Baess I. Report on a pseudolysogenic mycobacterium and a review of the literature concerning pseudolysogeny. Acta Pathol Microbiol Scand B Microbiol Immunol, 1971. 79(3): p. 428-34.
[3] Barksdale L and Arden SB. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu Rev Microbiol, 1974. 28(0): p. 265-99.
[4] Ackermann HW and Dubow MS. Viruses of prokaryotes. CRC Press, 1987.
[5] Ripp S and Miller RV. The role of pseudolysogeny in bacteriophage-host interactions in a natural freshwater environment. Microbiology (Reading), 1997. 143(6): p. 2065-2070.
[6] RippS and Miller RV. Dynamics of the pseudolysogenic response in slowly growing cells of Pseudomonas aeruginosa. Microbiology (Reading), 1998. 144 ( Pt 8): p. 2225-2232.
[7] Abedon ST. Disambiguating bacteriophage pseudolysogeny: an historical analysis of lysogeny, pseudolysogeny,and the phage carrier state. In Contemporary trends in bacteriophage research(ed. HT Aadms), 2009. pp 285-307. New York, NY: Nova Science Publisher.
[8] Mäntynen S, Laanto E, Oksanen HM, Poranen MM and Diaz-Munoz SL. Black box of phage-bacterium interactions: exploring alternative phage infection strategies. Open Biol, 2021. 11(9): p. 210188.
[9] Brathwaite KJ, Siringan P, Connerton PL and Connerton IF, Host adaption to the bacteriophage carrier state of Campylobacter jejuni. Res Microbiol, 2015. 166(6): p. 504-15.
[10] Liang L and Connerton IF. FlhF(T368A) modulates motility in the bacteriophage carrier state of Campylobacter jejuni. Mol Microbiol, 2018. 110(4): p. 616-633.
[11] Cenens, W., et al., Viral Transmission Dynamics at Single-Cell Resolution Reveal Transiently Immune Subpopulations Caused by a Carrier State Association. PLoS Genet, 2015. 11(12): p. e1005770.
[12] Staes I, Backer LE, Simones K, Lavigne R, Bernaerts K, and Aertsen A. Superinfection exclusion factors drive a history-dependent switch from vertical to horizontal phage transmission. Cell Rep, 2022. 39(6): p. 110804.
[13] Yang LH, Wang J, Lu SG, Zhong YH, Xong K, Liu XX, Liu B, Wang XX, Wang P and Le S. Temperature-dependent carrier state mediated by H-NS promotes the long-term coexistence of Y. pestis and a phage in soil. PLoS Pathog, 2023. 19(6): p. e1011470.
专家简介 Expert Profiles

张婷婷,贵州医科大学 教授。中国生物工程学会噬菌体技术专业委员会委员。研究方向为微生物病毒(主要包括噬菌体和真菌病毒)。主持国家自然科学基金、国家博士后基金和省级多项科研项目,并发表多篇学术型论文。
Tingting Zhang, Professor, Guizhou Medical University, China. Member of Phage Technology Committee of Chinese Society of Bioengineering. Her research interests are microbial viruses (mainly including phage and fungal viruses). She has presided over the National Natural Science Foundation of China, National Postdoctoral Foundation of China and many scientific research projects at the provincial level, and has published many academic papers.
