Interactions between stressors are additive, antagonistic, and synergistic, respectively, when the combined effect is the sum, less than the sum, and greater than the sum, of the individual effects working in the same direction [24] (see [25] for extended definitions). Although treatment success is more likely for synergistic effects, simple additive ones can also obtain it, particularly in in vivo contexts where the host’s immune system can be integrated as a third line of control [26]. We now have a wealth of examples of positive interactions between antibiotics and lytic phages in controlling bacterial pathogens both in vitro and in vivo (Table 1). For instance, phages combined with antibiotics can eradicate strains of Klebsiella pneumoniae that form biofilm (see Glossary) and arrest the emergence of resistant variants in vitro; these combinations also stop methicillin-resistant Staphylococcus aureus (MRSA) hind paw foot infection in diabetic mice, and completely protect chickens from Escherichia coli infections in broilers [17,27,28]. In these cases and others, the combination treatment of a lytic phage with an antibiotic resulted not only in better control or eradication of bacteria, but also in the complete prevention of the emergence of resistant variants, compared to single antimicrobial treatments (Table 1).
压力源之间的相互作用分为相加效应、拮抗效应和协同效应,当综合效应是同方向作用的单个效应之和、小于单个效应之和或大于单个效应之和时[24](扩展定义见[25])。虽然协同效应更有可能取得治疗成功,但简单的相加效应也能取得治疗成功,特别是在体内环境中,宿主的免疫系统可以作为第三道控制线[26]。我们现在有大量抗生素和噬菌体在体外和体内控制细菌病原体的良性互动实例(表 1)。例如,噬菌体与抗生素结合可根除形成生物膜(见术语表)的肺炎克雷伯氏菌菌株,并阻止体外抗药性变种的出现;这些组合还可阻止糖尿病小鼠后爪足部感染耐甲氧西林金黄色葡萄球菌(MRSA),并完全保护鸡免受肉鸡大肠杆菌感染[17,27,28]。在这些案例和其他案例中,与单一抗菌素治疗相比,溶菌噬菌体与抗菌素的联合治疗不仅能更好地控制或根除细菌,还能完全防止耐药变种的出现(表 1)。
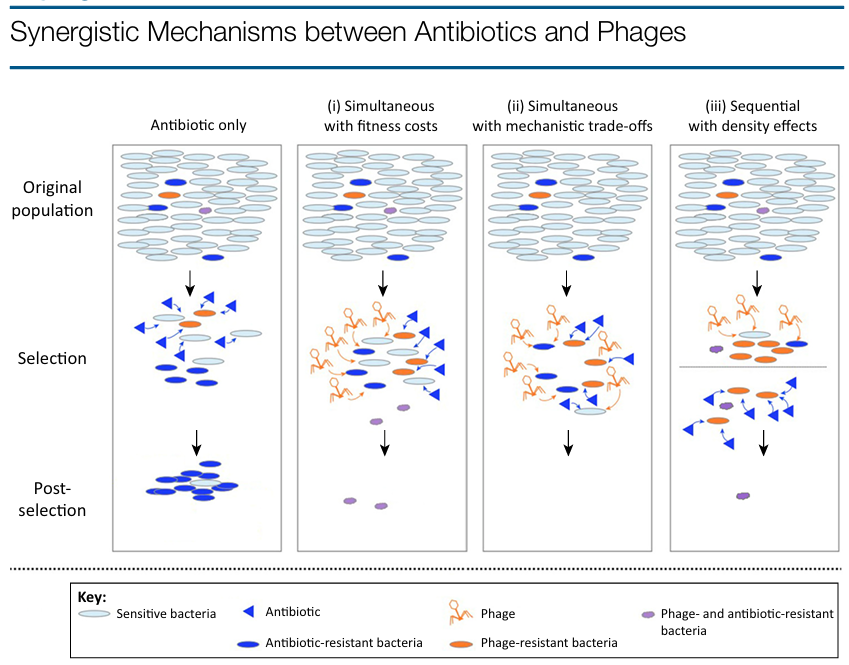
Darwinian evolution explains these positive outcomes in three non-mutually exclusive ways (Figure 1, Key Figure). First, bacteria may have genetic constraints, whereby the emergence of resistance mutations to one or both control agents is impeded by costs. An experimental example of this is the lower fitness of Pseudomonas fluorescens submitted to antibiotic–phage combinations compared to those evolved in single treatments [29]. Second, a direct negative interaction between mechanisms of resistance can be a powerful constraint on their evolution. For example, the antibiotic-induction of an aggregator phenotype in S. aureus increases survival to the antibiotic treatment, but predisposes the cells to phage predation (probably by increasing the numbers of phage receptors) [30]. This could also be the case for Salmonella bacteria resistant to phages that employ efflux pumps as receptors: phage resistance leading to decreased pump activity would increase sensitivity to antibiotics [31], although experimental evidence is needed to confirm this interaction. Third, synergy can result purely from low bacterial densities reducing the probability of the emergence of resistance mutations, as shown in the sequential application of antimicrobials to control P. aeruginosa [32]. Besides the logic of combining phages and antibiotics, phage therapy benefits from other qualities such as their aforementioned capacities to amplify in situ and evolve to overcome potential resistance, restricted host spectrum (e.g., minimizing impacts on the microbiome), the hypothetically unlimited number of phages available as alternatives should certain phages fail, and the assemblage of specific phage cocktails for each bacterial pathogen. Similar to the logic of phage cocktails, cocktails of antibiotics and phages designed and administered following an evolutionary rationale can reduce the development of resistance, especially in multiple bacterial infections, where some pathogenic species or clones could be favored should simple treatments eliminate competitors [33–35]. In summary, evolutionary theory provides a framework for understanding how two antimicrobial agents targeting different cellular processes and involving different resistance mechanisms can act synergistically to decrease bacterial densities and limit the emergence of resistance to either agent.
达尔文进化论以三种互不排斥的方式解释了这些积极的结果(图 1,关键图)。首先,细菌可能存在遗传限制,对一种或两种控制剂的抗性突变的出现受到成本的阻碍。这方面的一个实验例子是,与单一疗法相比,使用抗生素-噬菌体组合的假单胞菌的抗药性较低[29]。其次,抗药性机制之间的直接负作用可能会对它们的进化产生强有力的制约。例如,抗生素诱导的金黄色葡萄球菌聚集体表型可提高抗生素治疗的存活率,但却使细胞更容易被噬菌体捕食(可能是通过增加噬菌体受体的数量)[30]。沙门氏菌对噬菌体的抗性也可能是这种情况:噬菌体抗性导致泵活性降低,从而增加对抗生素的敏感性[31],尽管这种相互作用还需要实验证据来证实。第三,协同作用的产生可能纯粹是因为细菌密度低,降低了抗药性突变出现的概率,如在控制铜绿假单胞菌的过程中连续使用抗菌素[32]。除了噬菌体与抗生素相结合的逻辑之外,噬菌体疗法还具有其他优点,如上述的原位扩增能力和克服潜在抗药性的进化能力、有限的宿主谱(例如,最大限度地减少对微生物组的影响)、在某些噬菌体失效时可作为替代品的噬菌体的假设数量不受限制,以及针对每种细菌病原体组装特定的噬菌体鸡尾酒。与噬菌体鸡尾酒的逻辑类似,按照进化原理设计和施用抗生素和噬菌体鸡尾酒也能减少抗药性的产生,尤其是在多重细菌感染中,如果简单的治疗就能消灭竞争者,那么一些病原菌种类或克隆可能会受到青睐 [33-35]。总之,进化理论提供了一个框架,可用于理解针对不同细胞过程并涉及不同抗药性机制的两种抗菌剂如何协同作用,以降低细菌密度并限制对其中任何一种抗菌剂产生抗药性。
Key Figure 关键数据
Antibiotic-resistant bacteria
抗生素耐药细菌
Figure 1. Antibiotic resistance is strongly selected in antibiotic-only therapy (left panel). When phages and antibiotics are combined simultaneously (i) bacteria resistant to both antibiotics and phages may emerge, but grow slowly due to costs and/or are less pathogenic than sensitive bacteria, or (ii) double-resistant bacteria do not emerge due to trade-offs between resistance mechanisms. If phages and antibiotics are applied sequentially then (iii) double mutants are extremely rare or absent in the small populations remaining after the first antimicrobial agent is introduced and do not grow substantially thereafter. In general, bacteria resistant to only one agent are eliminated by the other, and during and following selection the immune system represents a third line of control.
图 1.在纯抗生素疗法中,抗生素耐药性被强烈选择(左图)。当噬菌体和抗生素同时使用时,(i) 可能会出现对抗生素和噬菌体都有抗药性的细菌,但由于成本原因生长缓慢,并且/或者致病性低于敏感细菌,或者 (ii) 由于抗药性机制之间的权衡,不会出现双重抗药性细菌。如果噬菌体和抗生素依次使用,那么(iii)双重突变体在引入第一种抗菌剂后剩余的小种群中极为罕见或不存在,此后也不会大幅增长。一般来说,只对一种抗菌剂产生抗药性的细菌会被另一种抗菌剂消灭,在选择过程中和选择后,免疫系统是第三道控制线。
Targeting Antibiotic-Resistant Bacteria
瞄准抗生素耐药细菌
As described above, combined phage–antibiotic therapies are a promising approach to con- trolling bacterial pathogens and limiting the evolution of resistance. However, accumulating research shows that under certain conditions, phage–antibiotic combinations can substantially impact populations of antibiotic-resistant bacteria. A prime example of this is the so-called phage–antibiotic synergy (PAS) phenomenon whereby antibiotics may stimulate the bacterial production of phages [36–41]. Of remarkable significance is the finding that sublethal doses of antibiotics produce the PAS effect. This means that regardless of the use of old or new antibiotics targeting multiresistant or naive bacteria, an antibiotic, when combined with phages, can potentially trigger a synergistic effect in reducing bacterial numbers [36,37,39]. The evolutionary reasoning for reducing antibiotic doses in clinical and environmental applications has been discussed elsewhere [42]. Concisely, evidence suggests that aggressive drug strategies maximize the evolutionary advantage of resistant pathogens [9,42,43]. The combination of sublethal doses of antibiotics with phages is a tantalizing alternative that can be both effective at reducing bacterial numbers and contribute to managing antibiotic resistance levels. A potential risk to the use of sublethal antibiotic doses is the activation of bacterial stress responses, such as the SOS response, that could increase survival and resistance to antibiotics (e.g., [44]). Additionally, horizontal dissemination of mobile elements, including virulence factors, and prophage protection against lytic phages are other clinically undesirable side effects of the activation of the SOS response [45,46]. Further investigation is needed to evaluate this concern, which may be prevented by using only certain types of antibiotics (avoiding, e.g., fluoroquinolones, b-lactams, and aminoglycosides) or combining antibiotics with specifically engineered phages that can block stress responses [44,47].
如上所述,噬菌体-抗生素联合疗法是控制细菌病原体和限制抗药性进化的一种很有前景的方法。然而,不断积累的研究表明,在某些条件下,噬菌体与抗生素的组合会对抗生素耐药细菌的数量产生重大影响。一个典型的例子就是所谓的噬菌体-抗生素协同作用(PAS)现象,即抗生素可刺激细菌产生噬菌体[36-41]。发现亚致死剂量的抗生素会产生 PAS 效应具有重要意义。这意味着,无论使用针对多重耐药菌还是幼稚菌的新旧抗生素,抗生素与噬菌体结合都有可能产生协同效应,减少细菌数量 [36,37,39]。在临床和环境应用中减少抗生素剂量的进化原理已在其他地方讨论过 [42]。简而言之,有证据表明,积极的药物策略能最大限度地提高耐药病原体的进化优势 [9,42,43]。亚致死剂量抗生素与噬菌体的结合是一种诱人的选择,既能有效减少细菌数量,又有助于控制抗生素耐药性水平。使用亚致死剂量抗生素的一个潜在风险是激活细菌的应激反应,如 SOS 反应,这可能会增加细菌的存活率和对抗生素的耐药性(如 [44])。此外,包括毒力因子在内的移动因子的水平传播以及噬菌体对溶解噬菌体的保护也是激活 SOS 反应在临床上产生的其他不良副作用 [45,46]。仅使用特定类型的抗生素(如避免使用氟喹诺酮类、b-内酰胺类和氨基糖苷类抗生素)或将抗生素与可阻断应激反应的特异性工程噬菌体结合使用,可避免这种副作用 [44,47]。
