Introduction 导言
While antibiotics have contributed to significant increased life expectancy in the last century1,2, the misuse and overuse of antibiotics jeopardize their effectiveness3,4. The emergence of antibiotic resistance and cross-resistance due to the evolutionary pressure by antibiotics is one of the major threats to human health, particularly to those with compromised immune systems3,5. The six leading pathogens that cause mortality associated with antimicrobial resistance are Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa6, and in 2019 these pathogens were responsible for over 250,000 worldwide deaths associated with antimicrobial resistance6.
尽管抗生素在上个世纪大大延长了人类的预期寿命 1,2 ,但抗生素的滥用和过度使用损害了其有效性 3,4 。抗生素的滥用和过度使用损害了其有效性 {{1} 。}.在抗生素的进化压力下,抗生素耐药性和交叉耐药性的出现是人类健康的主要威胁之一,尤其是对免疫系统受损的人来说 3,5 。与抗生素耐药性相关的六种导致死亡的主要病原体是大肠埃希菌、金黄色葡萄球菌、肺炎克雷伯菌、肺炎链球菌、鲍曼不动杆菌和铜绿假单胞菌 6 。2019 年,这些病原体导致全球超过 25 万人因抗菌药耐药性而死亡 6 。
Felix d’Hérelle first published the clinical use of bacteriophage (phage) therapy in 19213,7,8,9, and since then compassionate phage therapy has been applied to treat bacterial infections when standard, approved therapies fail10,11,12,13,14. The emerging antibiotic resistance pandemic has renewed attention in phage therapy, which uses lytic phages (viruses) that specifically infect bacteria and force them to switch their metabolism from growth to phage production3.
Felix d’Hérelle 于 1921 年首次发表了噬菌体疗法的临床应用 3,7,8,9 。从那时起,噬菌体疗法就被用于治疗细菌感染,当标准的、已获批准的疗法失效时 10,11,12,13,14 。噬菌体疗法利用溶解性噬菌体(病毒)特异性感染细菌,迫使细菌将新陈代谢从生长转为产生噬菌体 3 。
Phages are abundant in nature and within microbiomes, outnumbering bacteria worldwide by an estimated factor of 102,15, which suggests the ability to find phages for antibiotic resistant bacteria. In addition to this host-specificity, phages are self-amplifying, and have the ability to disrupt biofilms, which are important characteristics to target resistant, persistent bacterial infections3,16,17,18,19,20. However, despite numerous efforts in recent decades, the introduction of phages as approved drugs has been challenging due to phage diversity, narrow spectrum of activity, and challenges to produce a long-term, stable biologic21,22,23,24,25,26. In the U.S. phage therapy occurs via a single-patient investigational new drug (SPIND) application to the Food and Drug Administration (FDA) under the FDA’s expanded access IND (eaIND) program27,28. FDA eaIND review includes the clinical indication(s), protocol for phage therapy, phage manufacturing, and the proposed consent form. Individuals cannot be candidates for existing clinical trials, which is relevant as more clinical trials are available for phage therapy. Institutional Review Board (IRB) approval or an IRB waiver is required.
噬菌体在自然界和微生物群落中大量存在,其数量估计是细菌数量的 10 2,15 倍。这表明抗生素耐药细菌也能找到噬菌体。除了这种宿主特异性之外,噬菌体还具有自我扩增能力,并能破坏生物膜,这些都是针对耐药性、顽固性细菌感染的重要特征 3,16,17,18,19,20 。然而,尽管近几十年来做出了许多努力,但由于噬菌体种类繁多、活性谱狭窄以及生产长期稳定生物制剂的挑战,将噬菌体作为获批药物一直是个难题 21,22,23,24,25,26 。在美国,噬菌体疗法是根据食品与药物管理局(FDA)的扩大准入 IND(eaIND)计划,通过单病种研究性新药(SPIND)申请进入食品与药物管理局(FDA)的 27,28 。FDA eaIND 审查内容包括临床适应症、噬菌体疗法方案、噬菌体生产以及拟议的同意书。个人不能是现有临床试验的候选者,这与噬菌体疗法可进行更多临床试验有关。需要获得机构审查委员会 (IRB) 批准或 IRB 豁免。
This study focuses on the steps to develop phages for SPIND eaINDs using a phage targeting Pseudomonas aeruginosa. Pseudomonas aeruginosa is an opportunistic pathogen that is notoriously difficult to manage because it is frequently resistant to antibiotics29. While Pseudomonas aeruginosa is found in soil and water, it is an increasingly common human pathogen in immunocompromised patients, hospital-acquired or medical device infections, and in lung disease with impaired mucociliary clearance30,31. Pseudomonas aeruginosa requires antibiotics for treatment, and unfortunately the effectiveness of our current antimicrobial arsenal is dwindling due the ability of Pseudomonas aeruginosa to form biofilm and other mechanisms that increase the prevalence of antimicrobial resistance6. Antibiotics alone are increasingly insufficient in some patient cases, and adjuvant therapeutic strategies, such as phage therapy, are imperative for patients who do not respond to conventional medical approaches. Beyond phage therapy, other alternative methods for addressing antibiotic-resistant or recalcitrant Pseudomonas aeruginosa are being explored, such as vaccines, antimicrobial peptides, immunotherapy, iron chelators, and quorum sensing inhibitors31,32. However, none of these have been approved for clinical use to date.
本研究的重点是利用针对铜绿假单胞菌的噬菌体开发用于 SPIND eaINDs 的噬菌体的步骤。铜绿假单胞菌是一种机会性病原体,由于经常对抗生素产生耐药性 29 ,因此很难控制。虽然铜绿假单胞菌存在于土壤和水中,但在免疫力低下的患者、医院获得性感染或医疗器械感染以及粘膜纤毛清除功能受损的肺部疾病中,它是一种越来越常见的人类病原体 30,31 。铜绿假单胞菌需要使用抗生素进行治疗,遗憾的是,由于铜绿假单胞菌能够形成生物膜以及其他增加抗菌药耐药性的机制,我们现有抗菌药物的有效性正在下降 6 。在某些患者病例中,仅使用抗生素越来越不够,对于传统医疗方法无效的患者,噬菌体疗法等辅助治疗策略势在必行。除噬菌体疗法外,目前还在探索其他替代方法来治疗抗生素耐药或顽固的铜绿假单胞菌,如疫苗、抗菌肽、免疫疗法、铁螯合剂和法定量感应抑制剂 31,32 。然而,迄今为止,这些药物都尚未被批准用于临床。
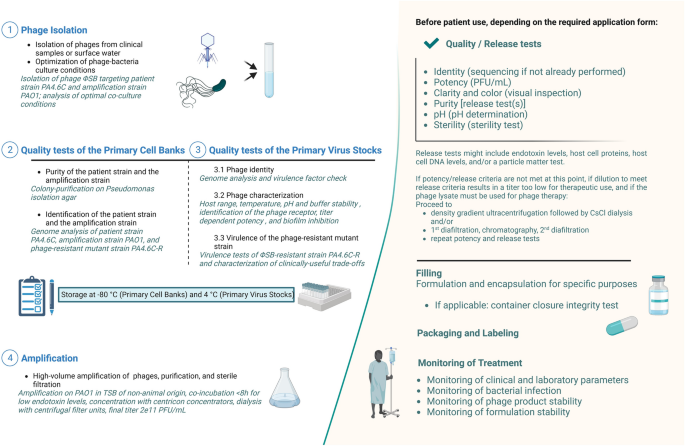
At Yale University’s Center for Phage Biology & Therapy, a preparatory modular approach has been established to produce safe and high-quality phages for SPIND eaINDs (Fig. 1), while recognizing that patient clinical condition may also affect time constraints. This pipeline starts from phage isolation (step 1) and includes a thoroughly characterized Primary Cell Bank (PCB, step 2), Primary Virus Stock (PVS, step 3), and production of a high-titer phage solution (step 4). Notably, we propose to include the evaluation of useful evolved trade-offs in phage-resistant bacteria because phage therapy exerts selection pressure for target bacteria to evolve phage resistance33. This analysis also excludes deleterious trade-ups, potentially caused by phage selection pressure22, as an integral part of the pipeline, which should be included in modern approaches to developing phage therapy34,35.
耶鲁大学噬菌体生物学与治疗中心(Center for Phage Biology & Therapy)建立了一套预备性模块方法,为 SPIND eaINDs 生产安全优质的噬菌体(图 1),同时考虑到患者的临床状况也可能影响时间限制。该流水线从噬菌体分离(第 1 步)开始,包括彻底鉴定原始细胞库(PCB,第 2 步)、原始病毒库(PVS,第 3 步)和生产高滴度噬菌体溶液(第 4 步)。值得注意的是,由于噬菌体疗法会对目标细菌产生噬菌体抗性 33 的选择压力,因此我们建议对噬菌体抗性细菌的有用进化权衡进行评估。这项分析还排除了噬菌体选择压力 22 可能导致的有害权衡。噬菌体选择压力 22 可能造成的损失性取舍,也是噬菌体疗法研发过程中不可或缺的一部分,应纳入噬菌体疗法的现代研发方法中 34,35 。