2.2. The Gut Microbiota–Brain Axis Network and Interdependence
2.2.肠道微生物群-大脑轴网络及相互依存关系
There is evidence that gut microbiota can also modulate functions of the nervous system and impact the maintenance of its homeostasis [86,87,88,89,90,91]. The link directly presenting this dependence is the vagus nerve, which maintains physical bidirectional communication between the CNS, intestinal wall, and enteric nervous system (ENS) [92], consisting of neurons and glial cells, distributed throughout the gastrointestinal tract and responsible for controlling coordinated smooth muscle contractile activity and other gut functions [93]. It has been suggested that the development and function of the ENS are to some extent mediated and modulated by gut microbiota [92]. Communication between them involves both direct and indirect pathways. The direct pathway includes altered intestinal permeability and relies on the release of signaling molecules into the gut lumen from immune and enterochromaffin cells. As a result, motor, sensory, and secretory modalities of the gastrointestinal tract increase [94,95,96]. Besides the ENS, those signaling systems consist of the endocrine-immune system, the hypothalamus–pituitary–adrenal (HPA) axis, and both the sympathetic and parasympathetic arms of the autonomic nervous system (ANS) [94,95,97]. Chemical signaling also occurs through a direct and indirect pathway. A well-reported direct pathway includes short-chain fatty acids (SCFAs), which are lipids produced in the fermentation of dietary fiber by microorganisms. They were proven to take part in regulating neuroplasticity, epigenetics, gene expression, and even immune response in CNS [98]. Other examples of chemicals produced by gut microbiota include hormones, such as corticotrophin-releasing hormone (CRH), serotonin, dopamine, and other neuropeptides [5,99,100,101]. It is also worth mentioning that it was demonstrated that increased uptake of fructose or exposure to SCFAs, such as acetic acid, increases the titer of phages specific to Lactobacillus reuteri and members of the genus Lactococcus [102]. The indirect impact includes modulating the neuroendocrine system [103]. An example of such regulation may include the production of glucagon-like peptide-1 (GLP-1), which was reported to increase appetite. Gut microbiota can also induce the production of neurotransmitters and regulate their concentration, which makes them mediators of classical signaling in the nervous system [104,105,106,107]. Moreover, some intestinal bacteria may also synthesize neurotransmitters—e.g., Bacteroides, Parabacteroides, and Escherichia spp. produce γ-aminobutyric acid (GABA) [104], which is the main inhibitory neurotransmitter in the human cortex [108]. As stated above, the gut phageome contains mostly CrAssphages specific to Bacteroides, thus it provides an extra layer of regulation in the production of these neurotransmitters. Research has also confirmed that the gut microbiota may suppress certain gut–brain signaling pathways through microbial metabolites. As for the immune response, the gut microbiota plays a crucial role not only in the proper modulation and functioning of the peripheral immune system but also in the maturation, development, and activation of microglia, which is essential for the innate immune response of brain cells [109,110]. The disruption of the bottom-up (gut-brain) motif can have a direct, substantial effect on the host’s mental state, potentially leading to depression, anxiety, and symptoms of bipolar disorder. It may also play a role in the pathogenesis, behavioral disturbance, and progression of neurodegenerative disorders, such as multiple sclerosis, Alzheimer’s disease, and Parkinson’s disease [19,111,112,113,114], as well as amyotrophic lateral sclerosis (ALS) [115] and epilepsy [116].
有证据表明,肠道微生物群还能调节神经系统的功能并影响其平衡的维持[86, 87, 88, 89, 90, 91]。直接呈现这种依赖性的环节是迷走神经,它维持着中枢神经系统、肠壁和肠道神经系统(ENS)之间的物理双向交流[92],由神经元和神经胶质细胞组成,分布于整个胃肠道,负责控制平滑肌的协调收缩活动和其他肠道功能[93]。有研究认为,ENS 的发育和功能在一定程度上受到肠道微生物群的介导和调节[92]。它们之间的交流涉及直接和间接途径。直接途径包括肠道通透性的改变,并依赖于免疫细胞和肠粘膜细胞向肠腔释放信号分子。因此,胃肠道的运动、感觉和分泌模式都会增加[94, 95, 96]。除 ENS 外,这些信号系统还包括内分泌-免疫系统、下丘脑-垂体-肾上腺(HPA)轴以及自主神经系统(ANS)的交感和副交感神经臂[94, 95, 97]。化学信号还通过直接和间接途径传递。短链脂肪酸(SCFAs)是微生物发酵膳食纤维时产生的脂质,是一种广为报道的直接途径。事实证明,它们参与调节中枢神经系统的神经可塑性、表观遗传学、基因表达甚至免疫反应[98]。肠道微生物群产生的其他化学物质包括激素,如促肾上腺皮质激素释放激素(CRH)、5-羟色胺、多巴胺和其他神经肽[5, 99, 100, 101]。值得一提的是,有研究表明,果糖摄入量的增加或接触醋酸等 SCFAs 会增加特异性雷特氏乳杆菌和乳球菌属噬菌体的滴度[102]。间接影响包括调节神经内分泌系统[ 103]。这种调节的一个例子可能包括胰高血糖素样肽-1(GLP-1)的产生,据报道它能增加食欲。肠道微生物群还能诱导神经递质的产生并调节其浓度,这使它们成为神经系统经典信号传递的媒介[104, 105, 106, 107]。此外,一些肠道细菌也可以合成神经递质–例如,Bacteroides、Parabacteroides 和 Escherichia spp.可以产生 γ-氨基丁酸(GABA)[104],而 GABA 是人类大脑皮层的主要抑制性神经递质[108]。 如上所述,肠道噬菌体组主要包含乳酸杆菌特有的 CrAssphages,因此它为这些神经递质的产生提供了额外的调节。研究还证实,肠道微生物群可能会通过微生物代谢物抑制某些肠道-大脑信号通路。至于免疫反应,肠道微生物群不仅在外周免疫系统的正常调节和运作中起着至关重要的作用,而且在小胶质细胞的成熟、发育和活化中也起着至关重要的作用,而小胶质细胞对脑细胞的先天性免疫反应至关重要[ 109, 110]。自下而上(肠道-大脑)模式的破坏会对宿主的精神状态产生直接、实质性的影响,可能导致抑郁、焦虑和躁狂症症状。它还可能在多发性硬化症、阿尔茨海默病和帕金森病等神经退行性疾病的发病机制、行为紊乱和恶化过程中发挥作用[19, 111, 112, 113, 114],也可能在肌萎缩性脊髓侧索硬化症(ALS)[115]和癫痫[116]中发挥作用。
On the other hand, the top-down (brain–gut) motif is based on the alteration of the gut by disturbed brain homeostasis. Scientists have pointed out several immunological and metabolic markers that are altered during neuroinflammation and infection [117]. It was suggested that if the gut–brain axis exists, then alterations in the CNS should be reflected in urine. Theoretically, during chronic CNS bacterial infection, the gut microbiome would be altered, thus leading to a perturbed metabolism both in the CNS and the gut, eventually releasing modified levels of metabolites or even new metabolites into the bloodstream. These metabolites would be filtered by the kidneys and, consequently, should be present in the urine. To confirm this hypothesis, the levels of three immunological biomarkers—interferon-gamma (IFNγ), vascular endothelial growth factor (VEGF), and myeloperoxidase (MPO)—were examined as their levels are altered during Mycobacterium tuberculosis meningitis [118,119]. The studies demonstrated elevated levels of all three biomarkers, indicating that the alteration in the gut–brain axis is indeed detectable in the urine [120,121,122].
另一方面,自上而下(大脑-肠道)模式的基础是肠道因大脑平衡紊乱而发生改变。科学家们指出,在神经炎症和感染期间,一些免疫和代谢标记物会发生改变[117]。有人认为,如果存在肠道-大脑轴,那么中枢神经系统的改变就应该反映在尿液中。从理论上讲,在中枢神经系统细菌慢性感染期间,肠道微生物组会发生改变,从而导致中枢神经系统和肠道的新陈代谢发生紊乱,最终向血液中释放出改变水平的代谢物,甚至是新的代谢物。这些代谢物会被肾脏过滤,因此会出现在尿液中。为了证实这一假设,研究人员检测了三种免疫生物标志物–γ 干扰素(IFNγ)、血管内皮生长因子(VEGF)和髓过氧化物酶(MPO)的水平,因为它们的水平在结核分枝杆菌脑膜炎期间会发生变化[118, 119]。这些研究表明,这三种生物标志物的水平都升高了,这表明肠-脑轴的改变确实可以在尿液中检测到[120, 121, 122]。
2.3. Naturally Occurring Bacteriophages in the Central Nervous System
2.3.中枢神经系统中自然存在的噬菌体
Bacteriophages inhabit various sites of the human body. It has been suggested that there is phage translocation from the intestinal tract to other parts of the body [123,124]. It has already been confirmed that they can enter through the epithelial cell barrier by transcytosis [13,125]. This evidence may provide a mechanism by which gut bacteriophages may access previously unknown body sites, such as the nervous system. Interestingly, phages have also been shown to penetrate neural endothelial cells [13]. Figure 1 presents the possible interactions of bacteriophages with the nervous system.
噬菌体栖息在人体的不同部位。有人认为,噬菌体可以从肠道转移到身体的其他部位[123, 124]。已经证实,噬菌体可以通过转囊作用进入上皮细胞屏障[13, 125]。这一证据可能为肠道噬菌体进入以前未知的身体部位(如神经系统)提供了一种机制。有趣的是,噬菌体还能穿透神经内皮细胞[13]。图 1 展示了噬菌体与神经系统可能发生的相互作用。
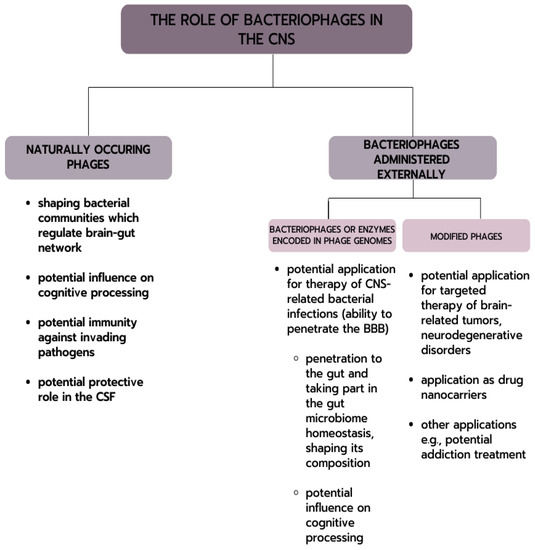
Figure 1. Possible phages interactions with CNS.
图 1.噬菌体与中枢神经系统可能的相互作用。
Surprisingly, in 2019, Ghose et al. identified viral particles in the cerebrospinal fluid (CSF) of healthy individuals (which at that time had been commonly considered sterile and unreachable) [28]. Examination of the CSF by epifluorescence microscopy demonstrated virus-like particles (VLPs) at an average of 104 per milliliter, yet other non-sterile human specimens such as saliva contained much more VLPs (106 VLPs/mL). Interestingly, attempts to amplify the V3-4 region of 16S rRNA in order to assess the CSF samples for the presence of any bacterial host were unsuccessful, suggesting that the CSF specimens were bacteria-free. Examination of sequencing reads from the CSF against currently available viral databases revealed that the reads had ~70% homology to known viral sequences, yet further characterization of the putative taxonomic composition determined that most of the contigs (~93%) could not be assigned to any known family, and of those that could be assigned, the vast majority represented tailed bacteriophages. These results suggest that the CSF and probably other parts of the nervous system may be populated by bacteriophages. However, it is still unknown whether these viruses serve a functional role as a non-host-derived immunity against potential bacterial invaders or if they simply entered the CSF with no apparent function [13,28,123,126,127]. Moreover, the effect that phages applied therapeutically may have on the functions of the nervous system has not been described so far.
令人惊讶的是,2019 年,Ghose 等人在健康人的脑脊液(CSF)中发现了病毒颗粒(当时人们普遍认为脑脊液是无菌的,无法触及)[ 28]。通过外荧光显微镜对脑脊液进行检查,发现平均每毫升脑脊液中含有 10 4 病毒样颗粒(VLPs),而唾液等其他非无菌人体标本中的 VLPs 含量要高得多(10 6 VLPs/mL)。有趣的是,试图扩增 16S rRNA 的 V3-4 区域以评估 CSF 样品中是否存在细菌宿主的尝试并不成功,这表明 CSF 样品中没有细菌。根据目前可用的病毒数据库对 CSF 的测序读数进行检查后发现,这些读数与已知病毒序列的同源性约为 70%,但对推测的分类组成进行进一步鉴定后发现,大多数等位基因(约 93%)无法归入任何已知的科,而在可以归入的等位基因中,绝大多数代表有尾噬菌体。这些结果表明,CSF 以及神经系统的其他部分可能存在噬菌体。然而,这些病毒是否具有抵御潜在细菌入侵者的非宿主免疫功能,或者是否只是进入脑脊液而没有明显的功能,目前还不得而知[13、28、123、126、127]。此外,噬菌体在治疗上的应用对神经系统功能可能产生的影响至今还没有描述。
As phages were confirmed to be naturally present in the nervous system, a new research field emerged to verify whether phages could be used in the treatment of bacterial diseases of the nervous system, such as meningococcal encephalitis, or—as modified bacteriophage—provide a safe carrier to be used in the context of treating cancers such as glioblastoma.
随着噬菌体被证实天然存在于神经系统中,一个新的研究领域应运而生,即验证噬菌体是否可用于治疗神经系统的细菌性疾病,如脑膜炎球菌性脑炎,或者作为改良噬菌体提供一种安全载体,用于治疗癌症,如胶质母细胞瘤。
